MODELLING OF TRANSIENT
RESPONSE FOR CATHETER – PRESSURE SENSOR SYSTEM
Vasile Manoliu
Depart. of Electrical Machines and Drive Systems, Faculty of
Electrotechnics, University “POLITEHNICA” of Bucharest, Splaiul Independentei
313 - 77206, Bucharest, Romania
Abstract Blood
pressure measurements are essential for control of circulatory systems with
artificial hearts or circulatory assist devices and are necessary in the
back-up monitoring of these systems. Extracorporeal pressure transducers
connected to a fluid-filled catheter have been most commonly used for short
term measurements of blood pressures in animal experiments of artificial
hearts/circulatory assist devices. With appropriate approximations on can
reduces a catheter-sensor lumped-parameter model to a second order system. In
this paper a transient response and the frequency response of the
catheter-sensor system by means of the analogous electric circuit is studied.
The effects of changes in the hydraulic system owing to air bubbles are, also,
considered.
1. INTRODUCTION
The heart is an extremely active organ, beating
approximately 30 million times, and ejecting some 2,1 million litres of blood,
per year. The cardiac cycle represents the events associated with the flow of
blood through the heart during one heart beat. Since alternate myocardial
contraction and relaxation mainly achieve the movement of blood, the cycle
employs the terms systole (i.e.
contraction) and diastole (i.e.
relaxation). Blood circulates because the heart “pump” establishes pressure
gradients.
An understanding of the dynamic properties of a
pressure-measurement system is important for preserve the dynamic accuracy of
the measured pressure. In the clinical situation errors in measurement of
dynamic pressure can have serious consequences (e.g. an underdamped system can
lead to overestimation of pressure gradients across stenotic heart valves).
Pressure sensors can monitor blood pressure in
postsurgical patients as part of a closed-loop feedback system. Such a system
injects controlled amounts of the drug nitroprusside to stabilize the blood
pressure.
The liquid-filled catheter sensor is a hydraulic
system that can be represented by either distributed- or lumped-parameters
models; for the clinical situation, the single-degree-of-freedom
(lumped-parameter) model is easier to work, and the accuracy of the results
obtained by using these models is acceptable.
2. HYDRAULIC – ELECTRICAL ANALOGY
In order to understand how the supply of blood to a
tissue is regulated in order to maintain cellular, tissue and organ system
homeostatic processes, on need to consider three interrelated physical aspects
of circulation: blood flow, blood pressure and peripheral resistance. The
latter two influence the rate of blood flow.
Blood circulates in the systemic and pulmonary
circuits, and the rate of flow is dependent upon two factors: arterial blood pressure and the peripheral resistance provided by blood
vessels and blood viscosity. The rate of flow is inversely proportional to the
resistance since for a given pressure, the higher the resistance, the lower the
flow rate.
Since the left ventricle pumps blood in a pulsating manner and tissue flow generally varies accordingly, the average pressure is very important. The difference between the blood pressure at the base of the aortic arch and the right atrium represents the circulatory pressure. Due to relatively small pressures in the venous system, arterial pressure approximates to the circulatory pressure.
Peripheral
resistance,
Rs, is the generally used term since most friction is encountered in
the peripheral circulation. Resistance is related to blood viscosity n, the length and the diameter of blood
vessels:
![]() (1)
(1)
The average pressure, ![]() , is determined only of cardiac
output CO and peripheral resistance:
, is determined only of cardiac
output CO and peripheral resistance:
![]() (2)
(2)
Cardiac output
is the
volume of blood ejected into the aorta each minute.
Arterial
compliance
determine only how quickly is obtained a new level of average pressure (i.e. time constant) when, for example, CO
is sudden modified.
The proportionality relation between cardiac output,
CO, and venous pressure, pv, is nonlinear:
![]() (3)
(3)
the proportionality factor, G, is named heart contractility.
A constant compliance model (or the linear three-element Windkessel
model) of arterial system is useful when pressure variation within the cardiac
cycle is small and at about normal mean blood pressure. When the mean blood
pressure is high, or when the pressure variation within a single cardiac cycle
is large, the nonlinear pressure dependent compliance model will more
accurately reflect the behavior of the arterial system.
Another parameter, proportional with blood density r, characterize uniform blood flow, is named inertance:
![]() (4)
(4)
with l and A length and cross-sectional area of
blood vessel, respectively.
Based on previous considerations, when the model is
represented with lumped parameters (circuit elements), can be used the
correspondence system between physiologic (hydraulic) and electrical elements,
presented in Table 1.
Table 1. Hydraulic-electrical
analogy
|
Hydraulic |
Electrical |
|
pressure p
[mm Hg ] |
voltage U [V] |
|
flow Q [ml / min] |
current I [A] |
|
flow resistance R
[mm Hg´min / ml] |
ohmic resistance
R [W] |
|
Compliance C
[ml /mm Hg] |
capacitance C [F] |
|
Inertance M
[g / cm4] |
inductance
L [H] |
|
Contractility G [ml /(min´mmHg)] |
conductance
G [W-1] |
|
Volume V [ml] |
charge q [C] |
3. ANALOGOUS
ELECTRIC SYSTEMS
The modeling approach taken here develops a
lumped-parameter model for the catheter and sensor separately.
Figure 1 shows the physical model of a
catheter-sensor system.
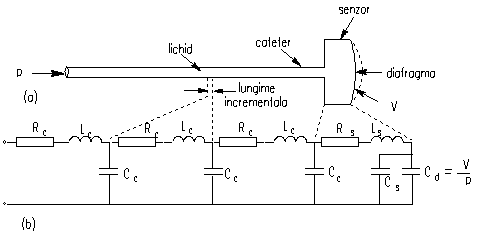
Fig. 1. a) Physical model of a
catheter–sensor system. b) Analogous electric system for this catheter–sensor
system.
An increase in pressure at the input of the catheter causes a flow of liquid to the right from the catheter tip, through the catheter, and into the sensor, this liquid shift causing a deflection of the sensor diaphragm, which is sensed by an electromechanical system. The subsequent electric signal is then amplified.
A liquid catheter has inertial, frictional and
elastic properties, represented by inertance, resistance and compliance,
respectively. Similarly, the sensor has the same properties, for the diaphragm
using in addition the compliance Cd. In fig. 1.b is shown an
electric analog of the pressure-measuring system.
The analogous circuit in Figure 1.b) can be
simplified to that shown in Figure 2.
Compliance of the sensor diaphragm, Cd,
is much larger than those of catheter or sensor cavity, provided that the
saline solution is bubble-free and the catheter material is relatively noncompliant. The resistance and
inertance of the liquid in the sensor can be neglected compared to those of the
liquid in the catheter.
The liquid resistance Rc of the catheter
is due to friction between shearing molecules flowing through the catheter;
Poiseuille’s equation can be used for calculate Rc knowing the
values of catheter length, its radius and the liquid viscosity.
The liquid inertance Lc of the catheter
is due primarily to the mass of the liquid.
Thus, on can neglect the resistive and inertial
components of the sensor with respect to those of the liquid catheter (the
liquid-filled catheter is longer than the cavity of the sensor and of smaller
diameter).
Using the analogy between input voltage and applied
pressure and applying the Kirchhoff’s voltage law in Figure 2, gives:
![]() (5)
(5)
where the compliance Cd of the sensor diaphragm
is the inverse of the volume modulus of elasticity of the sensor diaphragm.
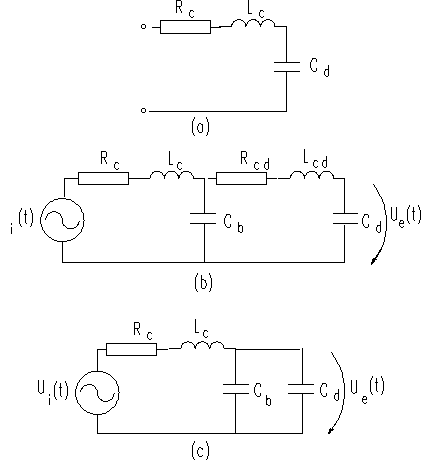
Fig. 2. a) Simplified analogous
circuit. b) Analogous circuit for catheter-sensor system with a bubble in the catheter. c) Simplified analogous circuit for catheter-sensor system
with a bubble in the catheter, assuming that Lcd
and Rcd are negligible with respect to Rc and Lc.
The natural undamped frequency is:
![]() (6)
(6)
and the damping ratio:
![]() (7)
(7)
By substituting in these relations the definition
expressions for Rc and Lc, can be shown that:
 (8)
(8)
and
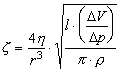 (9)
(9)
where:
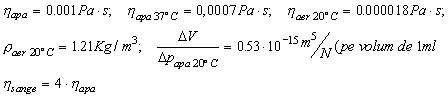
The transient response and the frequency response of
the catheter-sensor system by means of the analogous electric circuit can be
studied. In addition, on can study the effects of changes in the hydraulic
system by adding appropriate elements to the circuit. For example, an air
bubble in the liquid makes the system more compliant (catheter properties proximal to the bubble are resistance Rc and inertance Lc
and distal to the bubble: resistance Rcd and inertance Lcd). Thus its effect on the
system is the same as that caused by connecting an additional capacitor in
parallel to that representing the diaphragm compliance.
4. APPLICATION
A 5-mm-long air bubble has formed in the
rigid-walled catheter connected to a sensor. The catheter is 1m long, internal
radius is 0.46 mm and filled with water at 20°C; volume modulus of elasticity
of the diaphragm is 0,49×1015 N/m2. The isothermal
compression of air DV/Dp is 1ml per cm of water pressure per liter
of volume. On can obtain the frequency-response curve of the system with and
without the bubble.
The analogous circuit for the hydraulic system with
and without the bubbles is shown in Figure 2b) and c).
The values of natural frequency and the damping
ratio without the bubble is:
fn = 91 Hz and ![]() .
.
To calculate the new values of natural frequency and
the damping ratio for the case in which a bubble is present (5mm long), on
suppose the total compliance: Ct = Cd + Cb or:
![]() (10)
(10)
![]()
The volume of the bubble is:
![]()
One centimeter of water pressure is 98,5 N/m2.
Thus: DV/Dp = 3,38×10-14 m5/N
= Ct.
On can determine the new values for natural frequency
and damping ratio, assuming that the only parameter that changes is the value
of DV/Dp. Thus:
![]()
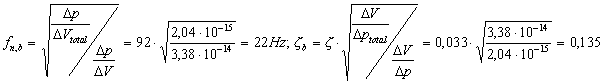
The frequency response for the system without
(continuous line) and with the bubble (circles line) present is shown in Figure
3.

Fig. 3. Frequency-response curves
for catheter-sensor system with and without bubbles.
Note that the bubble lowers fn and
increases z. This lowering of natural
frequency may cause distortion problems with the higher harmonics of the
blood-pressure waveform.
5. CONCLUSION
The measurement of blood pressures is essential for
control of circulatory systems with artificial hearts or circulatory assist
devices. In this paper was investigate a second-order system for a
catheter-sensor lumped parameter model. The presence of air bubbles may cause
distortion problems affecting information on arterial properties.
REFERENCES
[1] V. Manoliu – “Design and modelisation elements in bioengineering” (in romanian), U.P. Bucharest, 1999.
[2] J. G. Webster (ed.) – “Medical instrumentation – Application and design”, Ed. J. Wiley&Sons, 1998.